Clinical trials are essential for developing new drugs and therapies, but they are often slow, costly, and complex. The traditional process involves recruiting participants, managing extensive data, ensuring regulatory compliance, and analyzing outcomes—all of which can take years and significant resources. Artificial Intelligence (AI) is transforming clinical trials by making them faster, more efficient, and more accurate. AI algorithms can quickly analyze electronic health records (EHRs) to identify eligible participants, significantly reducing recruitment time and costs. During the trial, AI can monitor patients in real-time, using wearable devices and mobile apps to continuously collect data, detect adverse events early, and enhance patient safety.
Furthermore, AI excels in data analysis , uncovering patterns and insights that might be overlooked by traditional methods. This capability allows researchers to optimize trial designs, predict outcomes, and identify the most promising treatment approaches more effectively. This blog post explores the various ways AI is being used in clinical trials and the potential it holds for the future.
AI’s involvement in clinical trials spans multiple stages, from trial design to patient recruitment, data collection, monitoring, and analysis. The following sections detail how AI is integrated into each of these stages.
The design phase of a clinical trial is foundational, as it sets the framework for how the study will be conducted, what data will be collected, and how results will be analyzed. A well-designed trial can yield clear and reliable results, while a poorly designed trial can lead to ambiguous outcomes, wasted resources, and ethical concerns.
Simulation and Modeling: AI can simulate various trial scenarios using historical data, patient demographics, and disease progression models. This helps researchers optimize trial design by predicting potential outcomes and identifying the most effective approaches. AI can also accurately estimate the required sample size, preventing under- or over-enrollment, saving time and resources.
Predictive Analytics: AI analyzes historical trial data to identify patterns and predict challenges in upcoming trials. This allows researchers to tailor their designs, adjust inclusion criteria, and anticipate operational issues like recruitment bottlenecks or high dropout rates. AI can also predict potential safety concerns, enabling the development of proactive monitoring plans.
| Traditional Approach | AI-Enhanced Approach |
|---|---|
| Manual design and protocol setup | Automated simulation and modeling |
| Limited predictive capabilities | Enhanced predictive analytics |
| Time-consuming process | Accelerated design phase |
Patient recruitment is one of the most challenging and time-consuming aspects of clinical trials. Finding the right participants who meet specific trial criteria often involves sifting through vast amounts of data, which can delay the start of a trial and increase costs. AI offers a powerful solution to streamline this process.
Natural Language Processing (NLP): AI algorithms, especially those using NLP, can analyze electronic health records (EHRs) and other unstructured data to identify eligible patients based on detailed criteria. This reduces the time required to manually review records and increases the accuracy of patient selection by ensuring that all relevant data is considered.
Machine Learning Models: AI-driven machine learning models can predict patient eligibility by analyzing patterns in historical data. These models can assess patient engagement levels, helping to identify individuals who are not only eligible but also likely to adhere to the trial protocols. This improves retention rates and enhances the overall quality of the trial outcomes.
Virtual Trials: AI facilitates decentralized or virtual trials by enabling remote patient participation. This increases accessibility, allowing a more diverse and representative patient population to be included in trials, which is especially important in ensuring that results are generalizable across different demographics.
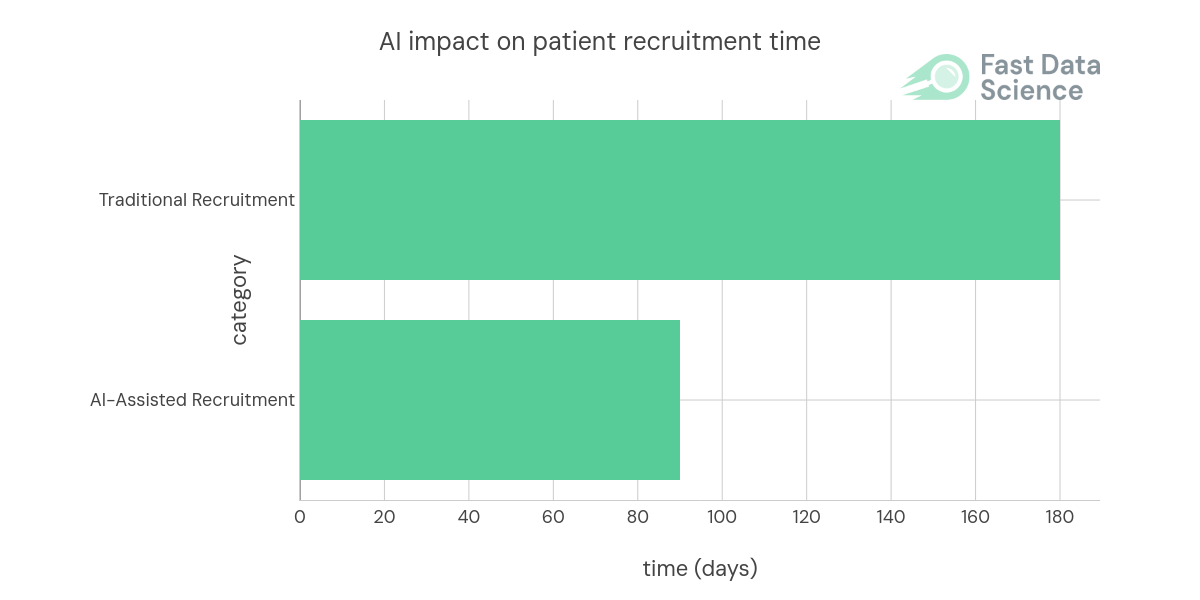
Graph 1: The use of AI can significantly reduce the time required for patient recruitment. Data source: IQVIA, AI in clinical development, Improving safety and accelerating results
Data collection is a crucial component of clinical trials, as the quality and accuracy of the data directly influence the trial’s outcomes. However, traditional data collection methods can be prone to errors, inconsistencies, and inefficiencies, often requiring frequent manual interventions. AI offers advanced solutions to enhance data collection and management, making the process more reliable and efficient.
Wearable Devices: AI-powered wearable devices, such as smartwatches and biosensors, enable continuous monitoring of patients throughout the trial. These devices can collect a wide range of data, including vital signs, physical activity levels, and even biochemical markers, all in real-time. This continuous data stream reduces the need for frequent clinic visits, improves patient compliance, and provides researchers with a more comprehensive and accurate picture of how patients are responding to the treatment.
Data Cleaning and Integration: One of the major challenges in clinical trials is the integration of data from multiple sources, including EHRs, laboratory results, patient-reported outcomes, and wearable devices. AI excels in data cleaning and integration by automatically identifying and correcting errors, inconsistencies, and missing values across datasets. This ensures that the data is accurate, consistent, and ready for analysis.
Automated Data Entry and Monitoring: AI-driven systems can automate data entry processes , reducing human errors associated with manual input. These systems can also monitor data entry in real-time, flagging any discrepancies or unusual patterns that may indicate potential issues. This not only improves data accuracy but also enhances the overall efficiency of the trial.
Monitoring patients during clinical trials is a critical aspect of ensuring both patient safety and the integrity of the trial’s results. Traditionally, this monitoring has relied on periodic manual checks, which can lead to delays in detecting adverse events and may not provide a comprehensive view of the patient’s condition. AI offers significant improvements in this area by enabling real-time monitoring and predictive modeling.
Real-Time Monitoring: AI algorithms are capable of continuously analyzing data from wearable devices, electronic health records (EHRs), and other monitoring tools. This real-time analysis allows for the immediate detection of anomalies, such as changes in vital signs or unexpected symptoms, which could indicate an adverse event. By monitoring patients continuously, AI can detect issues as they occur, rather than waiting for scheduled check-ups.
Predictive Modeling: AI doesn’t just respond to current data; it also predicts future risks. By analyzing patterns in patient data , such as changes in vital signs, biomarkers, or previous adverse events, AI can forecast the likelihood of future adverse events. Predictive models can identify high-risk patients and provide early warnings, allowing for proactive interventions.
Automated Alert Systems: AI can also be integrated with automated alert systems that notify healthcare providers when specific thresholds are crossed or when an adverse event is likely to occur. These systems can be customized to the specific needs of the trial, ensuring that alerts are meaningful and actionable, reducing the risk of alert fatigue.
Longitudinal Monitoring: AI enables continuous longitudinal monitoring, where patient data is tracked over the entire duration of the trial. This comprehensive monitoring can identify subtle trends and long-term changes that might be missed in periodic checks, providing a deeper understanding of the treatment’s impact on patients.
| Traditional Monitoring | AI-Enhanced Monitoring |
|---|---|
| Periodic manual checks | Continuous real-time monitoring |
| Delayed detection of adverse events | Immediate detection and alerting |
| Reactive response to issues | Proactive and predictive interventions |
The vast amounts of data generated during clinical trials are invaluable, but they also present significant challenges in terms of analysis and interpretation. Traditional methods of data analysis, while effective, can be time-consuming and may not fully capture the complexity of the data. AI offers advanced tools and techniques that enhance the ability to extract meaningful insights from this data, driving more accurate and comprehensive conclusions.
Statistical Analysis: AI algorithms can perform complex statistical analyses far more quickly and accurately than traditional methods. These algorithms can handle large datasets, run multiple models simultaneously, and optimize parameters in ways that would be infeasible manually. AI can also adapt to the evolving data landscape of a trial, refining its analyses as more data becomes available.
Pattern Recognition: One of AI’s greatest strengths is its ability to identify patterns and correlations that may not be immediately apparent through conventional analysis. Machine learning models can analyze data at a granular level, detecting subtle trends, interactions, and relationships that might otherwise go unnoticed. For example, AI can uncover complex interactions between variables, such as the influence of genetic factors on treatment response, leading to new hypotheses and discoveries.
Biomarker Discovery: Biomarkers—biological indicators of disease or treatment response—are critical in the development of personalized medicine. AI is particularly effective in biomarker discovery because it can analyze large datasets of genetic, molecular, and clinical data to identify potential biomarkers. By integrating various data types, AI can reveal biomarkers that are predictive of treatment outcomes, disease progression, or patient response. This accelerates the development of personalized medicine approaches, where treatments are tailored to the individual characteristics of each patient, improving the efficacy and safety of therapies.
One of the most transformative applications of AI in healthcare is its ability to detect cancer cells at an early stage, even before symptoms appear. This early detection is crucial, as it can significantly improve patient outcomes and increase the success rate of clinical trials focused on cancer treatments. AI’s advanced capabilities can be leveraged in clinical trials to enhance early cancer detection, leading to more effective interventions and personalized treatment strategies.
Early Screening: AI algorithms excel at analyzing medical images such as mammograms, CT scans, MRIs, and other radiological data to identify early signs of cancer that might be overlooked by human eyes. These AI systems are trained on vast datasets of annotated medical images, enabling them to recognize subtle patterns and anomalies indicative of early-stage cancer.
Genomic Analysis: Beyond imaging, AI can delve into the genetic and molecular levels by analyzing genomic data to detect mutations and abnormalities associated with cancer. AI-powered genomic analysis can identify specific genetic markers and mutations that indicate a predisposition to cancer or the presence of cancerous cells. This early identification of genetic risks allows researchers to stratify patients in clinical trials, ensuring that treatments are tested on individuals who are most likely to benefit from them.
Personalized Treatment Plans: AI’s ability to detect cancer at an early stage and analyze genetic data enables the creation of highly personalized treatment plans. By understanding the specific characteristics of a patient’s cancer, AI can help tailor treatment protocols that are more likely to be effective, reducing the trial-and-error approach often associated with cancer treatment.
Integration with Liquid Biopsies: AI can also enhance the efficacy of liquid biopsies—non-invasive tests that detect cancer-related biomarkers in blood samples. By integrating AI with liquid biopsy data, researchers can monitor cancer progression and response to treatment in real-time, providing valuable insights during clinical trials.

Graph 2: AI’s potential in detecting cancer cells at an early stage, leading to better trial outcomes. Data source: McKinney, Scott Mayer, et al. International evaluation of an AI system for breast cancer screening. Nature 577.7788 (2020): 89-94.
Regulatory submissions are a critical phase in the clinical trial process, as they determine whether a new drug or treatment can proceed to the next stage of development or reach the market. This phase requires the meticulous compilation of vast amounts of data, adherence to stringent regulatory standards, and thorough documentation. AI is increasingly being used to streamline this complex and time-consuming process, ensuring that submissions are both accurate and efficient.
Automate Documentation: The preparation of regulatory submission documents is traditionally a labor-intensive process, often requiring the input of multiple teams to ensure that all necessary information is included and correctly formatted. AI tools can automate much of this process by extracting relevant data, organizing it according to regulatory guidelines, and generating comprehensive reports.
Compliance Monitoring: Ensuring compliance with regulatory requirements throughout a clinical trial is essential to avoid delays and potential rejections during the submission phase. AI can be employed to continuously monitor trial activities, tracking adherence to protocols, patient consent procedures, data management practices, and safety monitoring requirements.
Data Validation and Integrity Checks: Regulatory bodies require that the data submitted is both accurate and reliable. AI can perform automated data validation and integrity checks, ensuring that all data points are consistent, complete, and free from errors. These AI systems can cross-check data from different sources, identify discrepancies, and flag them for review.
Standardization Across Submissions: Different regulatory bodies may have varying requirements for how data and documentation should be presented. AI can assist in standardizing submissions by automatically formatting documents according to the specific guidelines of each regulatory agency. This adaptability ensures that submissions meet the unique requirements of global regulatory bodies, facilitating smoother approvals across different regions.
Audit Trail Creation: AI can also assist in creating detailed audit trails that document every step of the data collection, analysis, and submission process. These audit trails are essential for demonstrating compliance during regulatory reviews and inspections. AI can automatically log all actions, changes, and decisions made during the trial, providing a clear and traceable record that can be easily reviewed by regulatory authorities.
While AI offers significant benefits in clinical trials, its implementation also presents several challenges and ethical considerations that must be carefully addressed to ensure the technology is used responsibly and effectively.
Data Privacy: Ensuring patient data privacy and security is paramount, especially when dealing with sensitive health information. AI systems often require access to large datasets, which can include personal health information (PHI) such as medical records, genetic data, and real-time monitoring data from wearable devices. The use of such data raises concerns about how it is stored, processed, and shared. Robust encryption, anonymization techniques, and strict access controls are essential to protect patient confidentiality.
Bias in AI Algorithms: AI models are only as good as the data on which they are trained. If the training data is biased or unrepresentative, the AI models can inherit and perpetuate these biases, leading to skewed or unfair outcomes. For example, if a dataset predominantly includes data from one demographic group, the AI might be less accurate when applied to other groups, leading to disparities in treatment recommendations or patient outcomes. It is essential to use diverse and representative datasets during the training phase to minimize bias.
Ethical Use of AI: The ethical use of AI in clinical trials goes beyond data privacy and bias. There are broader concerns about the potential for AI to dehumanize the patient experience or reduce the role of human judgment in clinical decision-making. While AI can process data and make recommendations at an unprecedented scale, the final decision should always involve human oversight, particularly in areas where clinical judgment and patient-centered care are critical.
Informed Consent: Another ethical consideration is ensuring that patients are fully informed about how AI will be used in the clinical trial. This includes understanding what data will be collected, how it will be used, and what potential risks are involved. Patients should have the opportunity to consent to the use of AI in their care, with clear explanations provided about the benefits and limitations of AI-driven interventions.
The future of AI in clinical trials is incredibly promising, with advancements on the horizon that could fundamentally reshape how trials are designed, conducted, and analyzed. These advancements have the potential to make clinical trials more efficient, inclusive, and personalized, ultimately accelerating the development of new treatments and improving patient outcomes.
AI-Driven Personalized Medicine: One of the most exciting prospects for AI in clinical trials is the shift towards fully personalized medicine. AI could enable trials to be tailored to the individual characteristics of each patient, including their genetic makeup, health history, and lifestyle factors. This approach would allow for the development of treatments that are specifically designed to work for each individual, rather than relying on a one-size-fits-all approach. In the future, AI could analyze vast amounts of genetic and molecular data to identify which patients are most likely to benefit from a particular treatment, enabling more precise and effective therapies.
Increased Adoption of Virtual Trials: As AI technology continues to evolve, virtual trials—where patients participate remotely—may become increasingly common. Virtual trials offer several advantages, including greater convenience for participants, reduced costs, and the ability to include a more diverse patient population. AI can facilitate these trials by enabling remote monitoring, data collection, and analysis, ensuring that trial data remains accurate and reliable even when patients are not physically present at a trial site.
AI-Enhanced Adaptive Trials: The future may see an increase in the use of adaptive trial designs, where AI continuously analyzes incoming data to modify the trial in real-time. This could involve adjusting dosages, changing participant groups, or even terminating ineffective treatments early. Such flexibility could make trials more ethical and efficient, reducing the number of patients exposed to less effective treatments while speeding up the discovery of successful interventions.
Global Collaboration and Data Sharing: AI could facilitate greater global collaboration and data sharing in clinical trials. By securely aggregating and analyzing data from multiple trials across different regions, AI can help identify broader trends and patterns, making research more comprehensive and inclusive. This global perspective can also accelerate the development of treatments for rare diseases by pooling data from a larger, more diverse population.
AI is undeniably transforming the landscape of clinical trials, offering numerous benefits that span the entire trial process, from improved trial design and patient recruitment to more efficient data management, monitoring, and analysis. These advancements are not only making clinical trials faster and more cost-effective but are also contributing to more accurate and reliable results, ultimately accelerating the development of new, life-saving treatments. However, as with any powerful technology, the adoption of AI in clinical trials is not without its challenges. Issues such as data privacy, security, and the potential for bias in AI algorithms must be carefully managed to ensure that AI is used ethically and effectively. Addressing these challenges is crucial to maintaining the integrity of clinical research and ensuring that AI-driven advancements benefit all patient populations.
As AI technology continues to evolve, its role in clinical trials is expected to expand even further, paving the way for more personalized and adaptive trial designs, virtual trials, and real-time data analysis. These innovations promise to revolutionize the drug development process, making it more efficient, inclusive, and patient-centered. By embracing AI and proactively addressing the associated challenges, the clinical trial industry can unlock new opportunities for innovation and progress. Ultimately, the integration of AI into clinical research holds the potential to significantly improve healthcare outcomes, offering faster access to new therapies and improving the quality of life for patients around the world.
Dive into the world of Natural Language Processing! Explore cutting-edge NLP roles that match your skills and passions.
Explore NLP Jobs
Fast Data Science appeared at the Hamlyn Symposium event on “Healing Through Collaboration: Open-Source Software in Surgical, Biomedical and AI Technologies” Thomas Wood of Fast Data Science appeared in a panel at the Hamlyn Symposium workshop titled “Healing Through Collaboration: Open-Source Software in Surgical, Biomedical and AI Technologies”. This was at the Hamlyn Symposium on Medical Robotics on 27th June 2025 at the Royal Geographical Society in London.
We presented the Insolvency Bot at the 4th Annual Conference on the Intersection of Corporate Law and Technology at Nottingham Trent University Dr Eugenio Vaccari of Royal Holloway University and Thomas Wood of Fast Data Science presented “A Generative AI-Based Legal Advice Tool for Small Businesses in Distress” at the 4th Annual Conference on the Intersection of Corporate Law and Technology at Nottingham Trent University

What is generative AI consulting? We have been taking on data science engagements for a number of years. Our main focus has always been textual data, so we have an arsenal of traditional natural language processing techniques to tackle any problem a client could throw at us.
What we can do for you