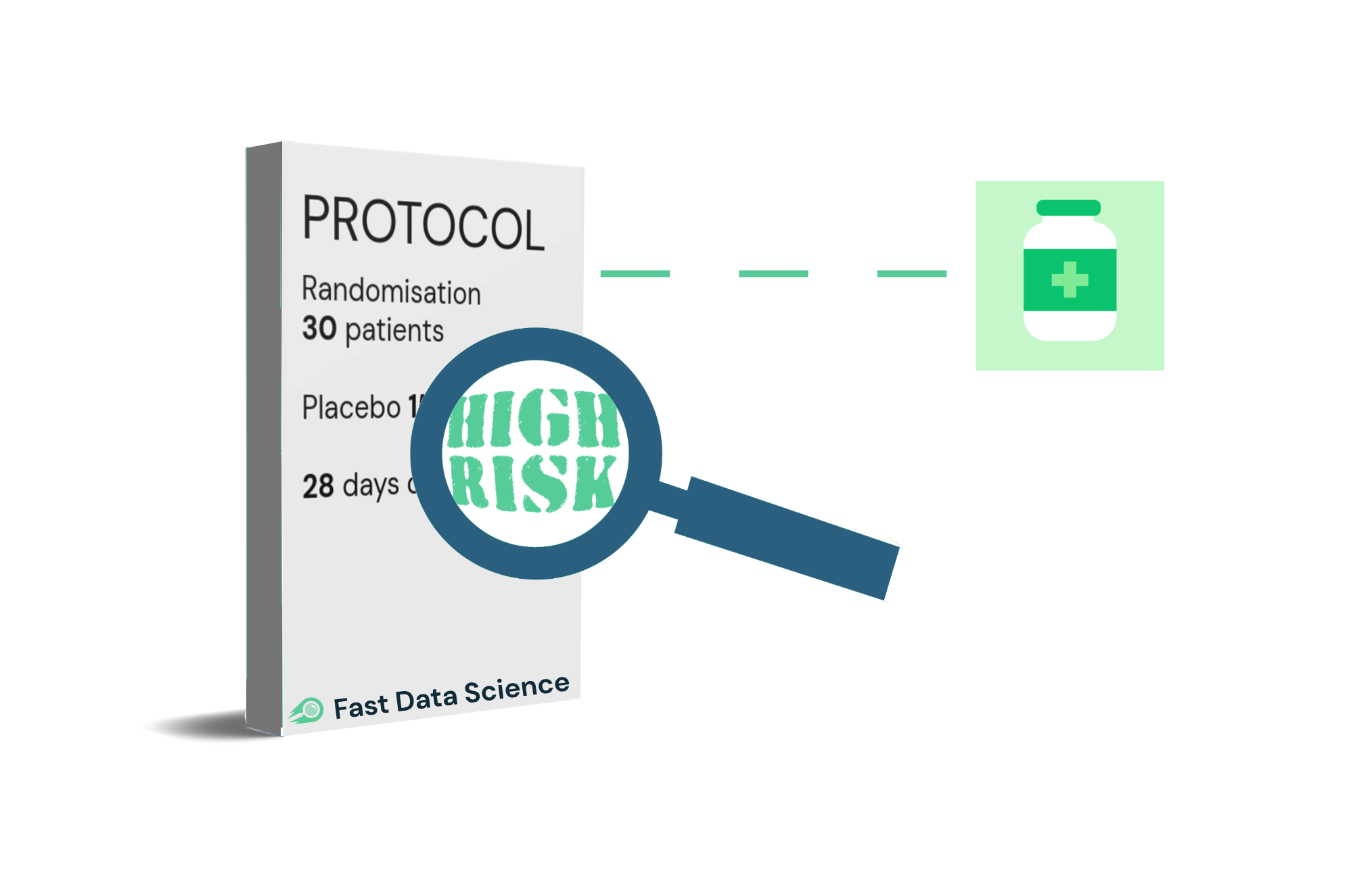
We developed a tool using Natural Language Processing for a client in the pharmaceutical space to assist experts to estimate the risk of a clinical trial ending uninformatively. You can read more about it in our guest article on Clinical Leader.
We were contacted by the Bill and Melinda Gates Foundation, who wanted a tool to assist reviewers in quantifying the risk of a clinical trial protocol. A protocol is a document, which is often in PDF format and which may be up to 200 pages long, containing a complete description of the plan of a trial: where it will take place, how many subjects will be recruited (the sample size), which interventions are to be tested, and how the statistical analysis is to be conducted.
Any organisation planning to fund a clinical trial must examine and stress-test the protocol thoroughly. The cost of running a trial is high and there are many points of potential failure. For example, if the sample size is too small, then the trial will not have sufficient statistical power to deliver an informative result and will not contribute to the body of knowledge of the funding organisation or the scientific community. This is called the risk of the trial ending uninformatively.
Protocols are written in technical English but are not constrained by any particular standard. Protocols from within a given organisation generally follow a rough pattern, but there are many ways that a particular data point can be communicated: the sample size could be referred to as the number of participants, N = 90, or the researchers could write simply we plan to enroll up to 100 subjects per site and leave it to the reader to infer the sample size.
The Gates Foundation needed an NLP model capable of quickly scanning a trial protocol and picking out key factors that could affect the risk of running the trial.
Over a period of more than a year, we experimented with an ensemble of machine learning and rule-based models to extract features such as the pathology, phase, sample size, number of countries, number of arms, presence or absence of a statistical analysis plan, effect size, and whether simulation had been used to determine the sample size. These parameters were put into a simple linear risk model and the tool generates a PDF or Excel report which can be shared within the organisation.
We deployed the tool to the internet at https://clinicaltrialrisk.org/tool and open-sourced the code under MIT licence.
The tool has enabled the funding organisation to assess incoming trials for rapid triage. It has also helped professionals worldwide to make a rough risk assessment of their trials before submitting them for funding.
If you would like to cite the tool alone, you can cite:
Wood TA and McNair D. Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness. Gates Open Res 2023, 7:56 doi: 10.12688/gatesopenres.14416.1.
A BibTeX entry for LaTeX users is
@article{Wood_2023,
doi = {10.12688/gatesopenres.14416.1},
url = {https://doi.org/10.12688%2Fgatesopenres.14416.1},
year = 2023,
month = {apr},
publisher = {F1000 Research Ltd},
volume = {7},
pages = {56},
author = {Thomas A Wood and Douglas McNair},
title = {Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness},
journal = {Gates Open Research}
}
Unleash the potential of your NLP projects with the right talent. Post your job with us and attract candidates who are as passionate about natural language processing.
Hire NLP Experts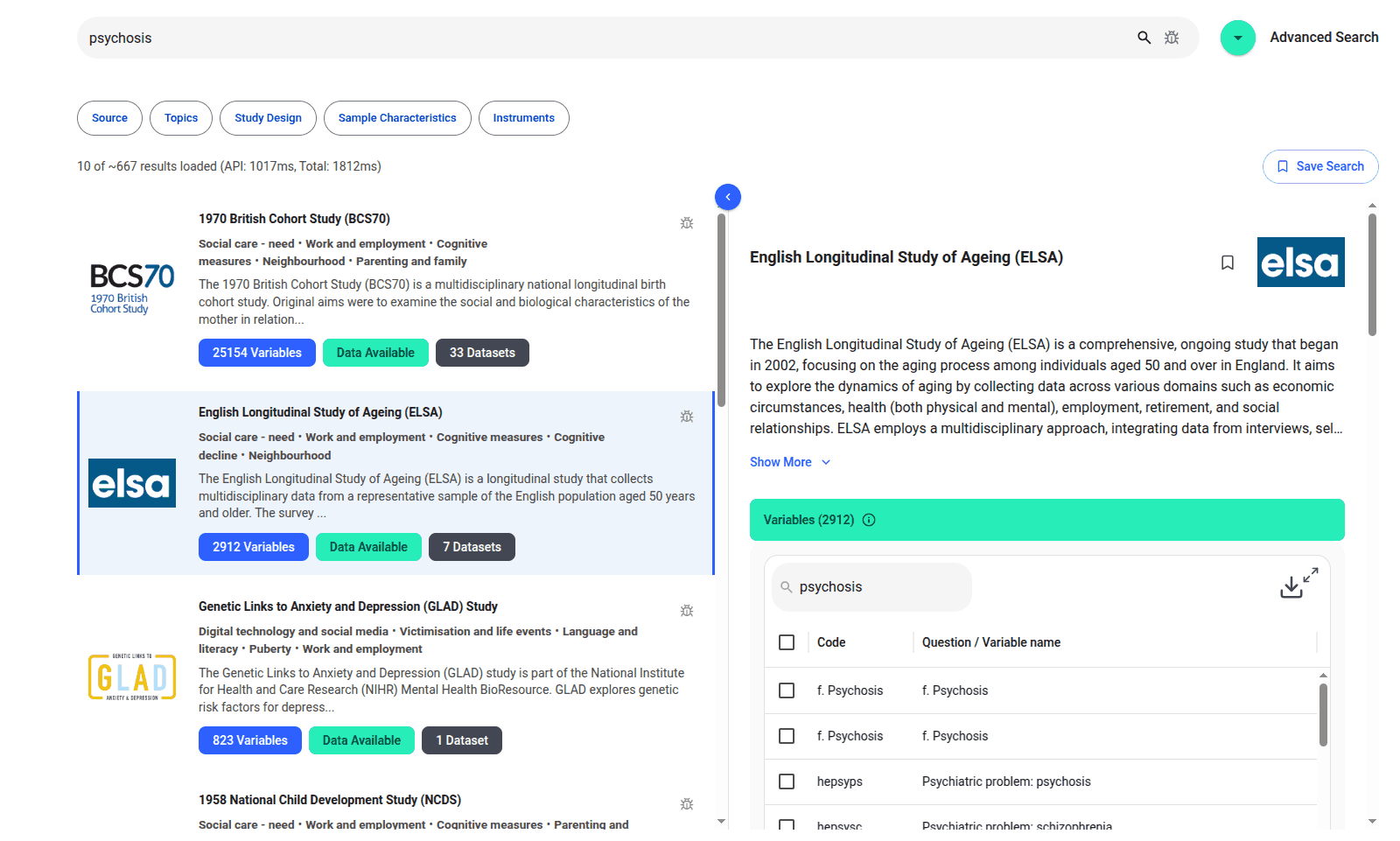
We are excited to introduce the new Harmony Meta platform, which we have developed over the past year. Harmony Meta connects many of the existing study catalogues and registers.

Guest post by Jay Dugad Artificial intelligence has become one of the most talked-about forces shaping modern healthcare. Machines detecting disease, systems predicting patient deterioration, and algorithms recommending personalised treatments all once sounded like science fiction but now sit inside hospitals, research labs, and GP practices across the world.
If you are developing an application that needs to interpret free-text medical notes, you might be interested in getting the best possible performance by using OpenAI, Gemini, Claude, or another large language model. But to do that, you would need to send sensitive data, such as personal healthcare data, into the third party LLM. Is this allowed?
What we can do for you