We’re proud to announce that the Clinical Trial Risk Tool has been selected as a winner of the Plotly Dash Example Apps Challenge (2023), out of 25 amazing apps submitted by the community!
You can try the tool at clinicaltrialrisk.org.
Natural language processing
Funded by the Bill and Melinda Gates Foundation, this app uses natural language processing, Plotly Dash, and the spaCy and Scikit-Learn libraries to calculate the risk of a clinical trial failing to deliver informative results. It reads the trial protocol and identifies key features from the text which are fed into a risk model.
Thomas Wood will be presenting the tool in a webinar on 7 June 2023 which you can book here.
Thank you to all #PlotlyCommunity members who participated in the recent #Dash Example Apps Challenge, and congratulations to the winning submissions!
— Plotly (@plotlygraphs) May 22, 2023
🥇 Clinical Trial Risk Dash App by Thomas Wood
🥈 SARIMA Tuner by Gabriele Albini
🥉 Product Environmental Report Dash App by…
Meanwhile, an article describing the tool has been published at: Wood TA and McNair D. Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness. Gates Open Res 2023, 7:56 (https://doi.org/10.12688/gatesopenres.14416.1).
Download the pitch deck for the Clinical Trial Risk Tool
If you would like to cite the tool alone, you can cite:
Wood TA and McNair D. Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness. Gates Open Res 2023, 7:56 doi: 10.12688/gatesopenres.14416.1.
A BibTeX entry for LaTeX users is
@article{Wood_2023,
doi = {10.12688/gatesopenres.14416.1},
url = {https://doi.org/10.12688%2Fgatesopenres.14416.1},
year = 2023,
month = {apr},
publisher = {F1000 Research Ltd},
volume = {7},
pages = {56},
author = {Thomas A Wood and Douglas McNair},
title = {Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness},
journal = {Gates Open Research}
}
Looking for experts in Natural Language Processing? Post your job openings with us and find your ideal candidate today!
Post a Job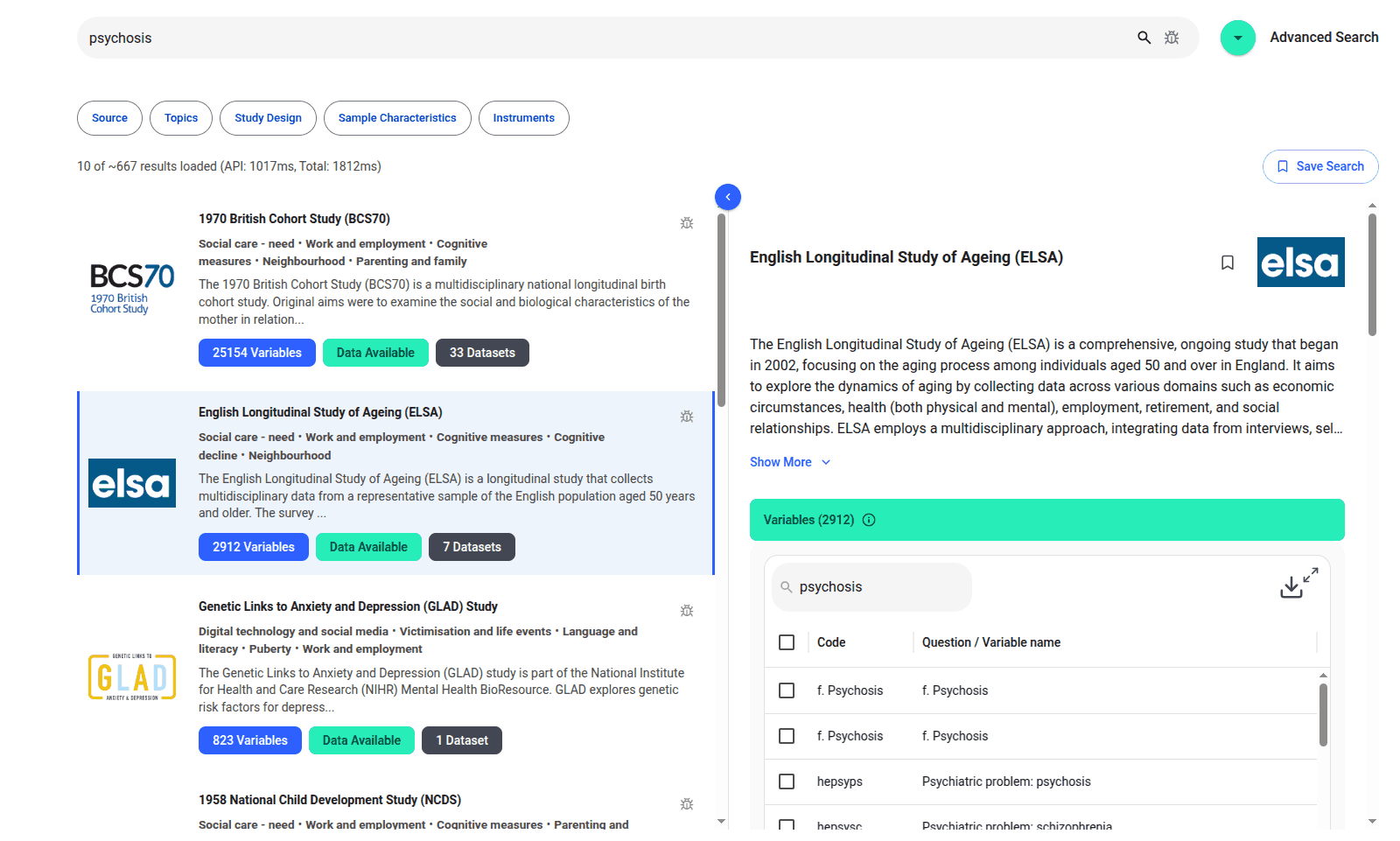
We are excited to introduce the new Harmony Meta platform, which we have developed over the past year. Harmony Meta connects many of the existing study catalogues and registers.

Guest post by Jay Dugad Artificial intelligence has become one of the most talked-about forces shaping modern healthcare. Machines detecting disease, systems predicting patient deterioration, and algorithms recommending personalised treatments all once sounded like science fiction but now sit inside hospitals, research labs, and GP practices across the world.
If you are developing an application that needs to interpret free-text medical notes, you might be interested in getting the best possible performance by using OpenAI, Gemini, Claude, or another large language model. But to do that, you would need to send sensitive data, such as personal healthcare data, into the third party LLM. Is this allowed?
What we can do for you