We’re proud to announce that the Clinical Trial Risk Tool has been selected as a winner of the Plotly Dash Example Apps Challenge (2023), out of 25 amazing apps submitted by the community!
You can try the tool at clinicaltrialrisk.org.
Natural language processing
Funded by the Bill and Melinda Gates Foundation, this app uses natural language processing, Plotly Dash, and the spaCy and Scikit-Learn libraries to calculate the risk of a clinical trial failing to deliver informative results. It reads the trial protocol and identifies key features from the text which are fed into a risk model.
Thomas Wood will be presenting the tool in a webinar on 7 June 2023 which you can book here.
Thank you to all #PlotlyCommunity members who participated in the recent #Dash Example Apps Challenge, and congratulations to the winning submissions!
— Plotly (@plotlygraphs) May 22, 2023
🥇 Clinical Trial Risk Dash App by Thomas Wood
🥈 SARIMA Tuner by Gabriele Albini
🥉 Product Environmental Report Dash App by…
Meanwhile, an article describing the tool has been published at: Wood TA and McNair D. Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness. Gates Open Res 2023, 7:56 (https://doi.org/10.12688/gatesopenres.14416.1).
Download the pitch deck for the Clinical Trial Risk Tool
If you would like to cite the tool alone, you can cite:
Wood TA and McNair D. Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness. Gates Open Res 2023, 7:56 doi: 10.12688/gatesopenres.14416.1.
A BibTeX entry for LaTeX users is
@article{Wood_2023,
doi = {10.12688/gatesopenres.14416.1},
url = {https://doi.org/10.12688%2Fgatesopenres.14416.1},
year = 2023,
month = {apr},
publisher = {F1000 Research Ltd},
volume = {7},
pages = {56},
author = {Thomas A Wood and Douglas McNair},
title = {Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness},
journal = {Gates Open Research}
}
Looking for experts in Natural Language Processing? Post your job openings with us and find your ideal candidate today!
Post a Job
Senior lawyers should stop using generative AI to prepare their legal arguments! Or should they? A High Court judge in the UK has told senior lawyers off for their use of ChatGPT, because it invents citations to cases and laws that don’t exist!

Fast Data Science appeared at the Hamlyn Symposium event on “Healing Through Collaboration: Open-Source Software in Surgical, Biomedical and AI Technologies” Thomas Wood of Fast Data Science appeared in a panel at the Hamlyn Symposium workshop titled “Healing Through Collaboration: Open-Source Software in Surgical, Biomedical and AI Technologies”. This was at the Hamlyn Symposium on Medical Robotics on 27th June 2025 at the Royal Geographical Society in London.
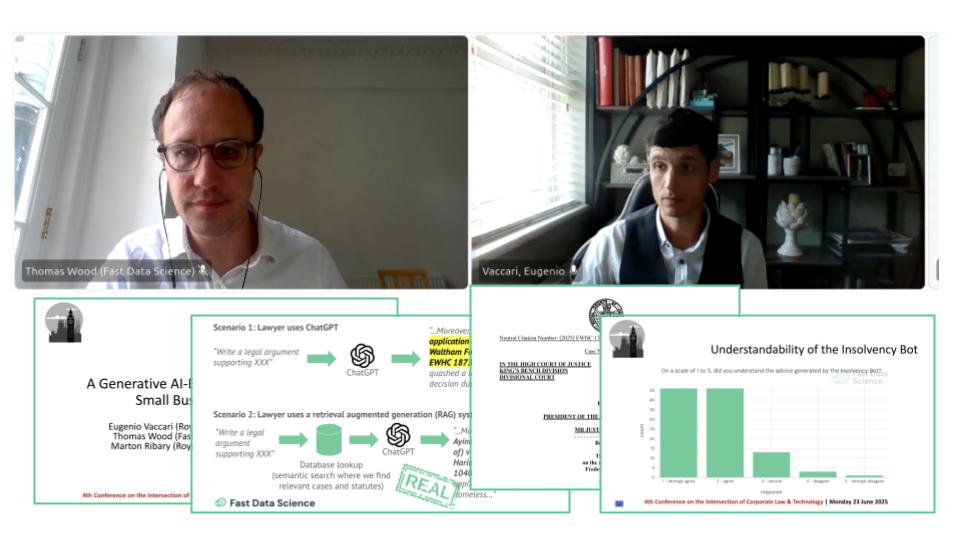
We presented the Insolvency Bot at the 4th Annual Conference on the Intersection of Corporate Law and Technology at Nottingham Trent University Dr Eugenio Vaccari of Royal Holloway University and Thomas Wood of Fast Data Science presented “A Generative AI-Based Legal Advice Tool for Small Businesses in Distress” at the 4th Annual Conference on the Intersection of Corporate Law and Technology at Nottingham Trent University
What we can do for you