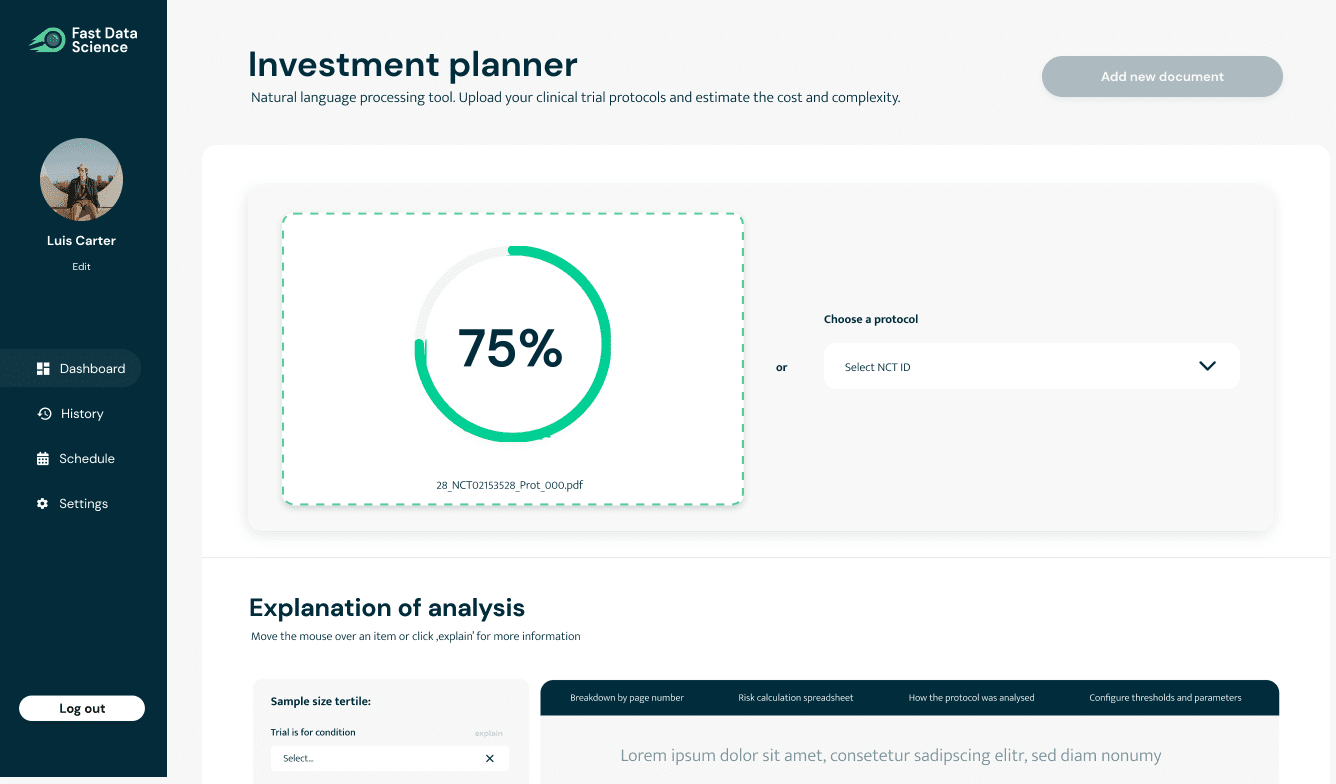
We are developing a natural language processing (NLP) tool, Fast Clinical AI, for identifying risk, cost, recruitment and enrolment criteria, and consent complexities of clinical trials using natural language processing, which can be integrated to data sources from trial management platforms, allowing pharma companies to leverage AI in their operations.
Clinical trial planning is currently a challenging area of drug development. A clinical trial is developed by a team of investigators with highly specialised expertise and written up as a protocol, often a PDF document of about 200 pages. Calculating the cost or risk of a clinical trial also requires a business assessment involving a team of highly skilled researchers. This is usually done manually inside pharma companies and funding organisations.
We have developed a set of tools using natural language processing (NLP) which can identify cost and risk factors in a protocol.
There is a first iteration of an earlier tool, the Clinical Trial Risk Tool, here, which has been featured by Gates Open Research and which won first prize in the Plotly Dash Challenge for its visualisation of an AI model in 2023.
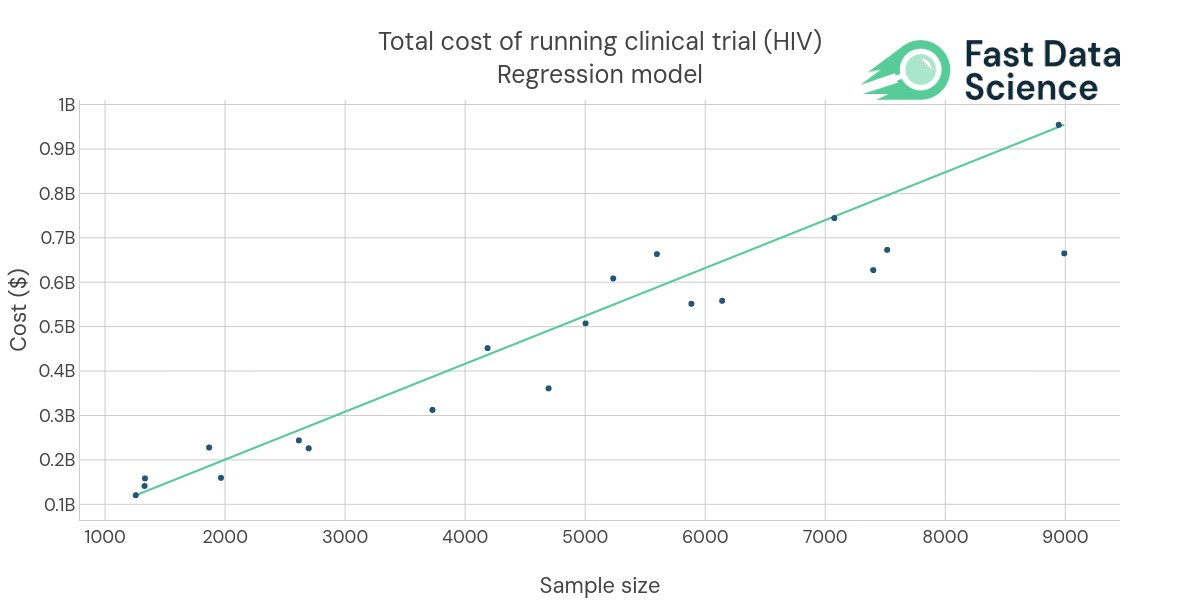
The current iteration of the Clinical Trial Risk Tool analyses HIV and TB clinical trial protocols and identifies risk factors using Natural Language Processing. You can upload a clinical trial protocol in PDF format, and the tool will generate a risk assessment of the trial.
AI in pharma
We are hoping to extend the existing prototype to further pathologies, in particular oncology trials, and add document management capabilities, customisable predictive models such as cost and risk modelling, and predictions based on enrolment criteria (such as anticipated difficulty of enrolment, or expected time needed to enrol the minimum sample size)
What is unique about our tool is that it uses cutting edge AI to extract key features from a protocol text, combined with traditional risk modelling or even manually determined heuristics to predict cost and other factors. This is termed a hybrid AI approach, and we are able to leverage this using a combination of natural language experience and pharma industry inside knowledge.
The tool does not simply extract features from a text, but crucially includes predictive modelling. We have found from prior consulting projects in this space that the addition of highly domain-specific predictive modelling (e.g. given this protocol PDF, what is the likely cost of a trial in $bn, or what is the likely enrolment time) allows organisations to control exposure to risk, improve their financial planning, and improve operational efficiency. For this reason we expect this tool to have a disruptive impact on the rather traditional way that many trials are currently designed.
Our prior consulting engagements by Boehringer Ingelheim and the Gates Foundation, as well as a number of smaller conversations and engagements with other companies in the pharma space, show that there is real interest and commercial drive to spend money on this kind of solution. We have built two very similar variants of this product in consulting engagements, so we anticipate strong demand once a robust product is in place.
The Clinical Trial Analysis Tool assists pharmaceutical companies and medical research organisations in planning, de-risking and optimising their clinical trials.
The tool helps optimise the process of bringing new drugs and devices from the laboratory to the market. Our project is focused on a data driven approach to clinical trial design and planning.
We hope that our tool will help to democratise trial development and boost the pharma industry in the UK and also in LMICs/the Global South. This will contribute to the UK’s standing as a leader in healthtech and medtech.
Download the pitch deck for the Clinical Trial Risk Tool
Our approach is innovative because, although there are a number of cost and risk metrics that exist, such as the Protocol Cost Tool developed by the WCG Metrics Champion Consortium/Avoca Quality Consortium, and TransCelerate’s Risk Assessment and Categorization Tool (RACT), these are often spreadsheets with dropdowns where a user can input up to about 100 key parameters of a trial. This is very different from the ease of use, efficiency and speed offered by a natural language understanding approach.
Our innovation can sit alongside the current assessment methods of clinical trials, but allows investigators, trial managers, and funders to run a quick triage of a trial to identify weak points, risks, or cost sinks, or to rapidly assess a bundle of trials or informed consent forms, such as those acquired from other pharma companies.
The tool will increase efficiency at the trial planning stage, and, if integrated into trial management software, allow for a feedback loop. This will ultimately bring benefits to the pharma industry, allowing cutting of costs (waste) and reduction of foreseeable risk and causes of trial failure, such as failure to enrol. Reduced clinical trial costs ultimately are passed onto patients and healthcare providers.
Although large language models (LLMs) and generative models such as GPT have recently become very mainstream, LLM based solutions often miss a crucial element of the puzzle which would drive value for pharma clients: a connection between the unstructured data in a clinical trial protocol, and likely outcomes of the trial (effect size, failure to enrol, failure to deliver informative results, cost in $Bn, etc). This latter problem can be addressed with predictive modelling and the combination with LLMs could be termed hybrid AI.
Our key technical innovation in Fast Clinical AI is the idea of a hybrid machine learning approach comprising: a natural language processing model processing the trial protocol text a risk, cost or other predictive model (customisable based on the user’s requirements), linked to the NLP model.
To our knowledge, no other product on the market is taking a hybrid approach to NLP combined with predictive modelling on clinical trial protocols.
Unleash the potential of your NLP projects with the right talent. Post your job with us and attract candidates who are as passionate about natural language processing.
Hire NLP Experts
We are excited to introduce the new Harmony Meta platform, which we have developed over the past year. Harmony Meta connects many of the existing study catalogues and registers.

Guest post by Jay Dugad Artificial intelligence has become one of the most talked-about forces shaping modern healthcare. Machines detecting disease, systems predicting patient deterioration, and algorithms recommending personalised treatments all once sounded like science fiction but now sit inside hospitals, research labs, and GP practices across the world.
If you are developing an application that needs to interpret free-text medical notes, you might be interested in getting the best possible performance by using OpenAI, Gemini, Claude, or another large language model. But to do that, you would need to send sensitive data, such as personal healthcare data, into the third party LLM. Is this allowed?
What we can do for you