Clinical trials are the backbone of medical progress, but a worrying trend is emerging: a large portion end without delivering useful results. This “uninformativeness” wastes valuable resources and delays advancements.
Fast Data Science is excited to announce the publication of a technical research paper the Clinical Trial Risk Tool, a game-changer in identifying potential uninformativeness at the protocol stage!
The Clinical Trial Risk Tool is a browser-based tool which uses Natural Language Processing (NLP) to analyse clinical trial protocols. Here’s how it works:
Ready to fight uninformativeness? Head over to https://clinicaltrialrisk.org/tool and access this free, open-source software.
A BibTex citation is as follows:
@article{wood2023clinical,
title={Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness},
author={Wood, Thomas A and McNair, Douglas},
journal={Gates Open Research},
volume={7},
number={56},
pages={56},
year={2023},
publisher={F1000 Research Limited}
}
Read more about research in AI and Fast Data Science’s publications here
Looking for experts in Natural Language Processing? Post your job openings with us and find your ideal candidate today!
Post a Job
None of the content of this article constitutes investment advice. Few would dispute that the global economy is currently in an AI boom. However, opinions are divided over whether this boom also constitutes a bubble.
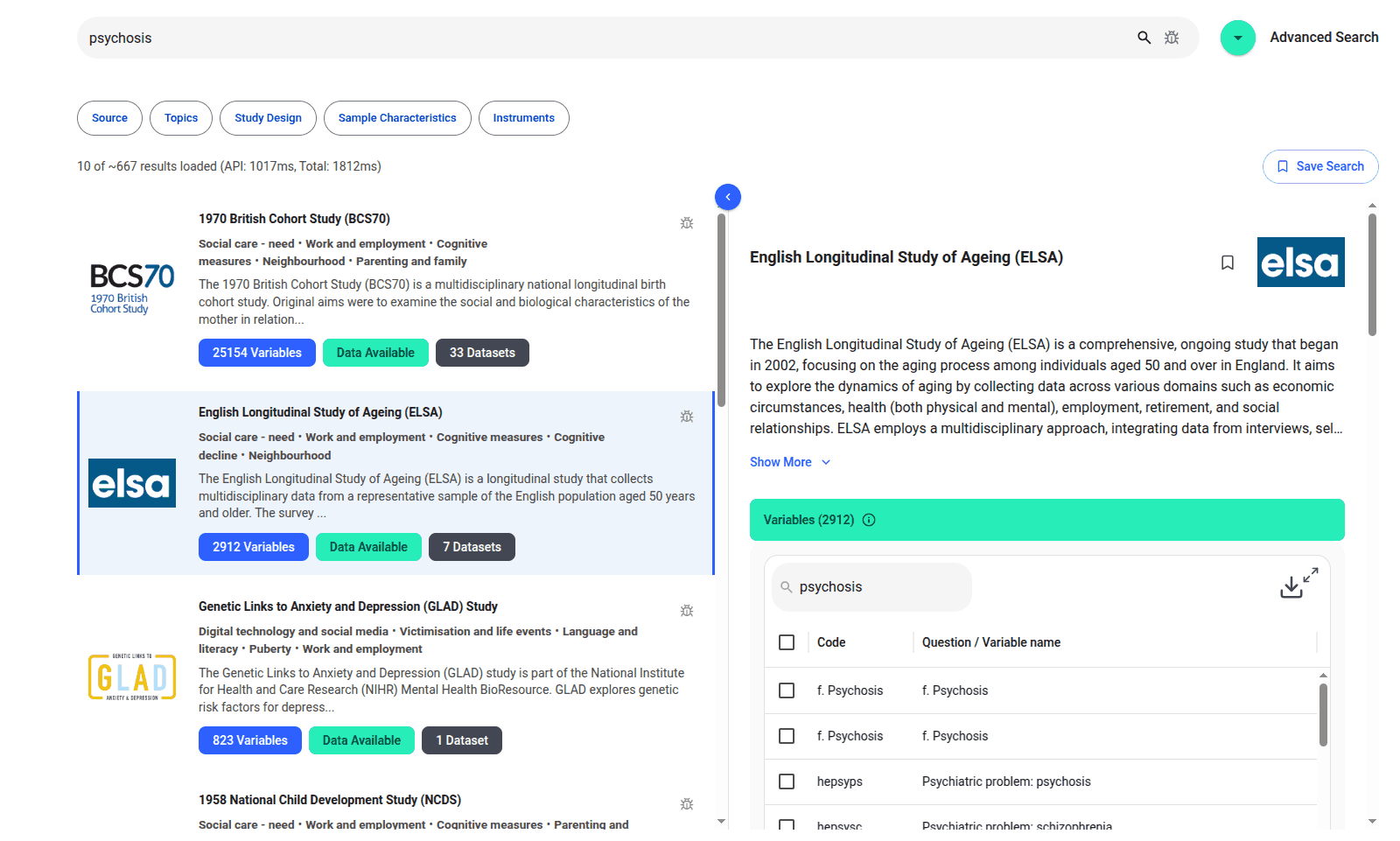
We are excited to introduce the new Harmony Meta platform, which we have developed over the past year. Harmony Meta connects many of the existing study catalogues and registers.

Guest post by Jay Dugad Artificial intelligence has become one of the most talked-about forces shaping modern healthcare. Machines detecting disease, systems predicting patient deterioration, and algorithms recommending personalised treatments all once sounded like science fiction but now sit inside hospitals, research labs, and GP practices across the world.
What we can do for you